Current and Emerging Treatments
Currently, dupilumab and nemolizumab are the only FDA-approved drugs for the treatment of prurigo nodularis (PN). Besides these, physicians typically prescribe treatments used for other skin disorders ranging from topical creams to systemic molecules that alter the immune response.1 Off-label therapies have limited efficacy and safety, making treatment extremely challenging.2, 3
Standard therapies include both pharmacologic and behavioral therapies. Behavioral treatments include ways to prevent itching including trimming fingernails, wearing long-sleeved clothes and gloves, appropriately bandaging lesions, adequate skin cleansing and moisturizing, and the use of anti-itch lotions including calamine, menthol, and camphor. Topical corticosteroids are the first type of treatment, followed by the use of antihistamines. Additional therapies can include phototherapy, immunosuppressants, and the use of antidepressants/anticonvulsants. Psychotherapy and relaxation technique also some ways to address the psychological effects of PN.1
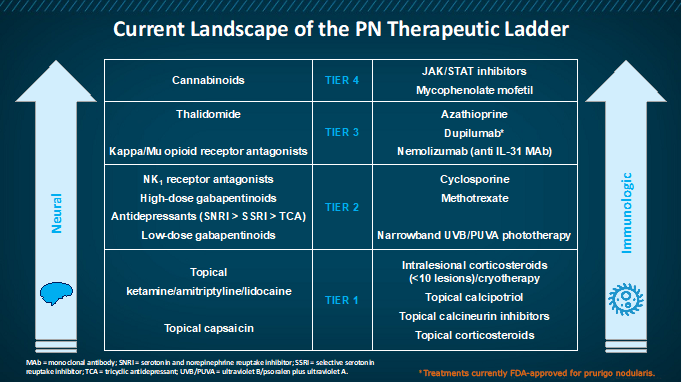
Table: Current treatments for prurigo nodularis4
Topical corticosteroids (TCSs) and topical calcineurin inhibitors (TCIs)
This class of drugs is largely used as first-line therapy. The mechanism of action of TCSs is via steroid receptors in the cytoplasm of the cells promoting an anti-inflammatory response. TCIs act by downregulating T-cell activity with a possible direct effect on nerve fiber function.5
Phototherapy
Ultraviolet A1 (UVA1) therapy is effective in a variety of inflammatory skin disease and acts by inducing T-lymphocyte apoptosis, reducing the number of Langerhans cells and mast cells in the dermis and reducing pruritis by interfering with histamine release from basophils and mast cells.5
Thalidomide
The antipruritic effects of thalidomide include neuromodulation and immunomodulation. The damage it causes to peripheral nerves may also contribute to its antipruritic effect.5 Its toxicity profile makes it less favorable for treating PN.6
Methotrexate
Methotrexate is a folic acid antagonist with immunomodulatory activity. Adverse events that are likely with methotrexate include nausea, fatigue, mucositis, diarrhea, skin rashes, and acute kidney injury.7
Two studies have highlighted its efficacy in PN:
- A retrospective review of 13 patients with treatment-resistant PN taking 7.5 mg to 20 mg of methotrexate weekly for 6 months demonstrated a ≥75% reduction in PN lesions and itching in 10 of 13 patients.7
- A study of 39 patients with PN taking 5 mg to 25 mg of methotrexate weekly showed an improvement in lesions in 91% of patients and an improvement in itching in 89% of patients.7
Pregabalin
An anticonvulsant with analgesic properties, pregabalin possibly acts by inhibiting voltage-dependent calcium channels in the dorsal root ganglion and spinal cord, thereby increasing the threshold for pruritic stimuli. 5
Antidepressants
A number of selective serotonin reuptake inhibitors (SSRIs) including paroxetine, sertraline, or fluvoxamine may be effective in pruritis. Amitriptyline has demonstrated efficacy in patients with neuropathic pruritis. Most agents in this class may be used as adjuvant therapies.8
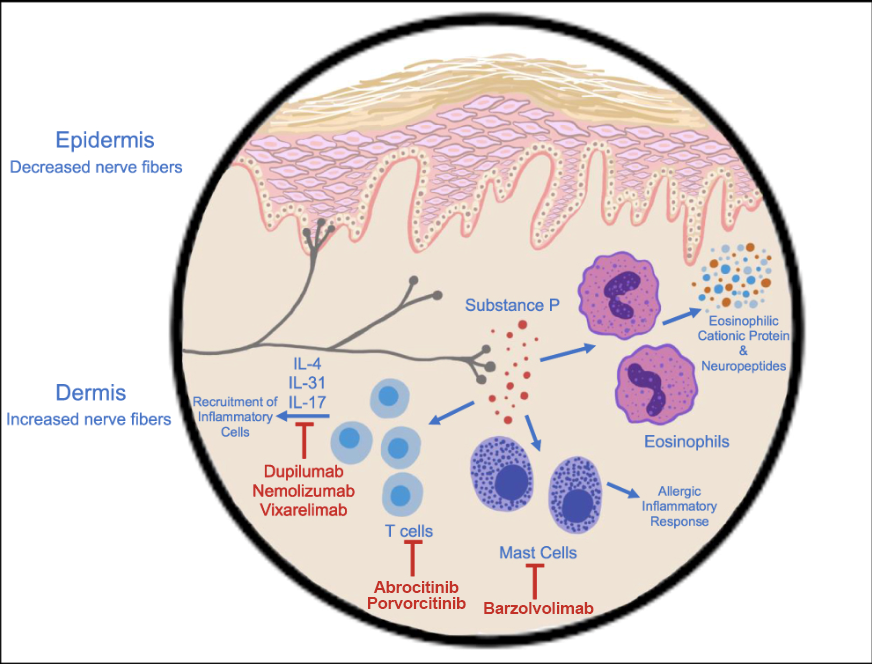
Figure: Targets of various immunotherapies7
NK1R antagonist aprepitant
Aprepitant is a neurokinin receptor 1 (NK1R) antagonist and acts by inhibiting the actions of substance P in peripheral and central itch signaling.5
Serlopitant is another NK1R antagonist that was studied for the treatment of PN, but it did not meet the primary objectives in 2 phase 3 trials, thus discontinuing its development for this indication.9
Nemolizumab
Nemolizumab is a subcutaneous (SC) administered humanized anti-IL-31 receptor monoclonal antibody currently approved for the treatment of itch associated with atopic dermatitis in Japan (only when existing treatment is not sufficiently effective).10 A 12-week phase 2 randomized, placebo-controlled trial (RCT) with 70 patients, of which 34 received SC nemolizumab, measured pruritis using the Numerical Rating Scale (NRS) at 12 weeks. A significant reduction in NRS was seen in patients on nemolizumab (-53% change) compared with placebo (-20.2% change). Nemolizumab received FDA-approval for the treatment of prurigo nodularis in August 2024.12
Highlights from the phase 3 OLYMPIA 2 trial include:
- 56% of patients on nemolizumab achieved a response in itch intensity (at least a 4-point improvement in peak-pruritis NRS score compared with 21% patients on placebo)12
- 38% of patients on nemolizumab reached treatment success in skin lesions compared with 11% of patients on placebo 12
- Side effects included gastrointestinal (GI) (namely, abdominal pain and diarrhea) and musculoskeletal symptoms (arthralgia, back pain, muscle spasm, jaw pain, fibromyalgia, spinal pain)13
Dupilumab
Dupilumab is a fully human monoclonal antibody that blocks the shared receptor component for IL-4 and IL-13, and the first drug FDA-approved for the treatment of PN in the United States. FDA approval was based on data from 2 clinical trials, PRIME and PRIME2, which evaluated the efficacy and safety of a 300 mg dose of dupilumab compared with placebo in adults with PN, with the primary goal of assessing the proportion of patients with a clinically significant reduction in itch or clearing of skin, or both.14
Highlights14 include:
- Data from PRIME show that 3 times as many patients on dupilumab (60%) experiencing a clinically significant reduction in itch from baseline at 24 weeks compared with 18% for placebo
- Data from PRIME2 show that 37% of patients on dupilumab experienced a clinically significant reduction in itch from baseline at 12 weeks compared with 22% for placebo
- More than twice as many patients on dupilumab (48% and 45%) achieved clear or almost clear skin at the end of 24 weeks as compared with patients on placebo (18% and 15.9%)
- More than 3 times as many patients on dupilumab (39% and 32%) experienced both a clinically significant reduction in itch and clear/almost clear skin at the end of 24 weeks, as compared with placebo (9% and 9%)
- Most common adverse events in the pooled PRIME and PRIME2 data in patients treated with dupilumab were nasopharyngitis, conjunctivitis, herpes infection, dizziness, muscle pain, and diarrhea
Opioids
Nalbuphine
A synthetic opioid, nalbuphine is a dual acting μ-antagonist and Κ-agonist that has shown efficacy in morphine-induced pruritis and uremic pruritis.
Highlights from a phase 2 RCT with an open-label extension phase include:
- Patients were treated with 81 mg or 162 mg nalbuphine extended-release (NAL-ER) tablets twice daily or placebo for 10 weeks15
- 44.4% of patients treated with NAL-ER 162 mg and 27.3% of patients treated with NAL-ER 81 mg achieved greater than or equal to a 30% reduction from baseline in 7-day Worst Itch NRS at Week 10 versus 36.4% in the placebo group15
- Adverse events were mild to severe15
Nalbuphine is no longer being developed for the treatment of prurigo nodularis.
Naloxone and naltrexone are 2 other opioids that are used as off-label treatment of PN.16
Cannabinoids
Cannabinoid receptor agonists have the potential of diminishing histamine-induced excitation leading to itch reduction. Further research is required to ascertain their role in the treatment of PN.4
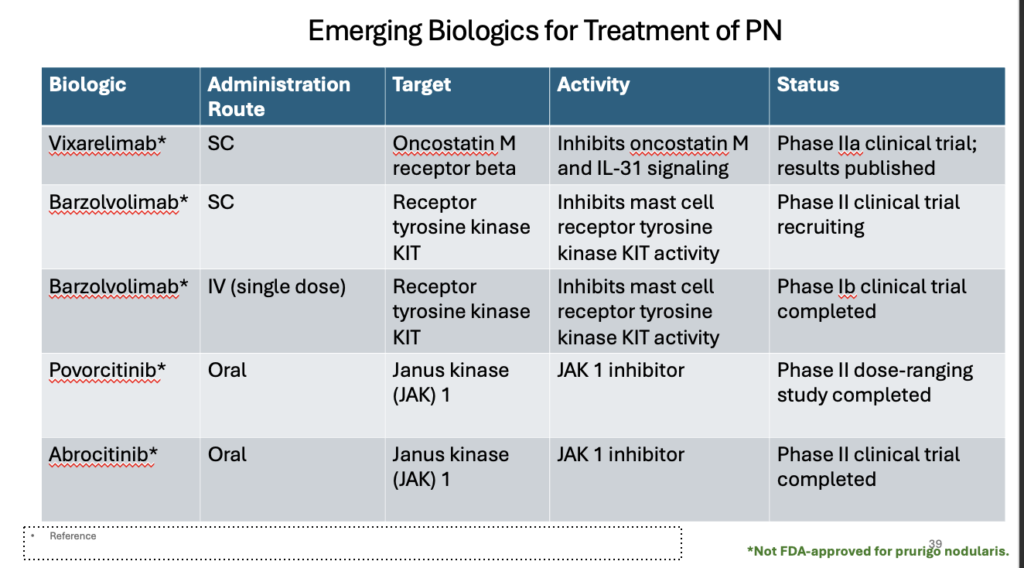
Other novel therapies in development
Vixarelimab, an oncostatin M (OSM) beta receptor antagonist, works by inhibiting the IL-31 pathways and is currently being evaluated for its efficacy in PN in a phase 2 clinical trial (NCT03816891). It demonstrated an average reduction of itch in 70% of patients following 8 weeks of treatment, with significantly improvement of nodules. Adverse effects were mild and transient. 7,11
Barzolvolimab is a tyrosine kinase (KIT) receptor inhibitor in clinical trials for the treatment of PN.7,11
Abrocitinib and povorcitinib, Janus kinase 1 (JAK1) inhibitors are 2 other molecules that are in phase 2 clinical trials for the treatment of PN. 7,11
Apremilast, a phosphodiesterase (PDE4)-inhibitor, is also in clinical trials for the treatment of PN (NCT03576287).11
Table: Emerging treatments for prurigo nodularis17
References
- National Organization for Rare Disorders (NORD). Prurigo nodularis. Updated 2/13/2023. https://rarediseases.org/rare-diseases/prurigo-nodularis/. Accessed 6/13/2023.
- Williams KA, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14(1):67-77.
- Satoh T, et al. 2020 guidelines for the diagnosis and treatment of prurigo. J Dermatol. 2021;48(9): e414-431.
- Elmariah S. et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84(3):747-760.
- Frølunde AS, et al. Non-atopic chronic nodular prurigo (Prurigo nodularis Hyde): a systematic review of best-evidenced treatment options. Dermatology. 2022;238:950-960.
- Kowalski EH, et al. Treatment-resistant prurigo nodularis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:163-172.
- Labib A, et al. Immunotargets Ther. 2022;11:11-21.
- Yosipovitch G, et al. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol. 2018;142:1375-1390.
- BioSpace. Menlo’s serlopitant for prurigo nodularis itching flunks two phase 3 trials. https://www.biospace.com/article/menlo-s-serlopitant-for-prurigo-nodularis-itching-flunked-2-phase-iii-trials/. Accessed 6/13/2023.
- Keam SJ. Nemolizumab: first approval. Drugs. 2022;82(10):1143-1150.
- US National Library of Medicine. Clinical trials. https://beta.clinicaltrials.gov/.
- Medscape Medical News. https://www.medscape.com/viewarticle/fda-approves-nemolizumab-prurigo-nodularis-2024a1000ewo?form=fpf. Accessed 11/22/2024.
- Ständer S, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020; 382:706-716.
- Yosipovitch G, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Weisshaar E, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermat Venereology. 2022;36(3):453-461.
- Mullins TB, et al. Prurigo nodularis. StatPearls. National Library of Medicine. Updated 9/12/2022. https://www.ncbi.nlm.nih.gov/books/NBK459204/. Accessed 6/13/2023.
- US National Library of Medicine. Clinical trials. https://beta.clinicaltrials.gov/. Accessed 9/11/2023.